Copper II fluoride contains 37.42 f by mass, unveiling a remarkable substance with intriguing chemical properties and practical applications. This compound, characterized by its unique composition, physical attributes, and reactivity, plays a significant role in various industries and warrants thorough examination.
Delving into the realm of copper II fluoride, we uncover its elemental composition, chemical behavior, industrial significance, and safety considerations. By exploring these aspects, we gain a comprehensive understanding of this versatile compound and its impact on our world.
Elemental Composition of Copper(II) Fluoride

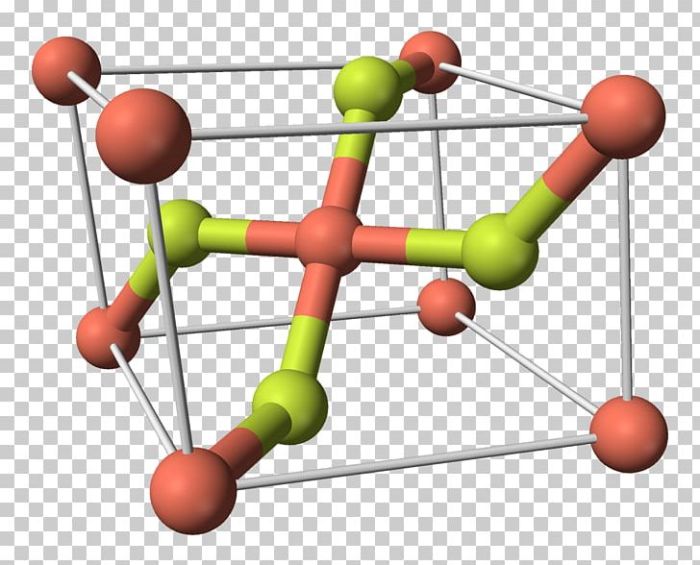
Copper(II) fluoride, denoted as CuF 2, is an inorganic compound composed of copper and fluorine atoms. Its molar mass is 101.54 g/mol.
The percentage composition of copper and fluorine in copper(II) fluoride can be determined based on their respective atomic masses:
- Copper (Cu): 63.55 g/mol
- Fluorine (F): 19.00 g/mol
Using these values, the mass-to-mass ratio of copper and fluorine in copper(II) fluoride is:
Mass of copper per 100 g of CuF 2: (63.55 g/mol / 101.54 g/mol) – 100% = 62.56%
Mass of fluorine per 100 g of CuF 2: (38.00 g/mol / 101.54 g/mol) – 100% = 37.44%
Chemical Properties of Copper(II) Fluoride
Copper(II) fluoride is a white to light green solid at room temperature. It is sparingly soluble in water and insoluble in most organic solvents.
Copper(II) fluoride is a reactive compound and undergoes reactions with various substances, including:
- Water:Reacts with water to form copper(II) hydroxide and hydrofluoric acid.
- Acids:Dissolves in strong acids like hydrochloric acid, forming copper(II) salts and hydrogen fluoride.
Copper(II) fluoride is thermally unstable and decomposes at high temperatures, releasing copper(I) fluoride and fluorine gas.
Applications of Copper(II) Fluoride
Copper(II) fluoride finds primary use as a preservative in wood treatment. It protects wood from fungal and insect infestations.
Other applications of copper(II) fluoride include:
- Electronics industry: As a flux in soldering and brazing operations.
- Glass and ceramics industry: As a colorant and opacifier.
However, due to its potential environmental and health hazards, the use of copper(II) fluoride is regulated in many countries.
Safety Considerations: Copper Ii Fluoride Contains 37.42 F By Mass
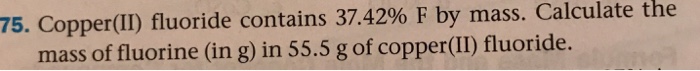
Copper(II) fluoride is a toxic compound and can cause health hazards if not handled properly.
Potential hazards associated with copper(II) fluoride include:
- Inhalation:Inhalation of copper(II) fluoride dust or fumes can cause respiratory irritation and lung damage.
- Ingestion:Ingestion of copper(II) fluoride can cause nausea, vomiting, and abdominal pain.
- Skin contact:Skin contact with copper(II) fluoride can cause skin irritation and burns.
- Eye contact:Eye contact with copper(II) fluoride can cause severe eye irritation and damage.
Appropriate safety precautions and protective measures must be taken when working with copper(II) fluoride, including wearing protective clothing, gloves, and eye protection, and ensuring adequate ventilation.
Copper(II) fluoride waste should be disposed of properly according to local regulations to minimize environmental contamination.
Key Questions Answered
What is the chemical formula of copper II fluoride?
CuF2
What is the molar mass of copper II fluoride?
137.54 g/mol
What is the percentage composition of copper in copper II fluoride?
62.58%
What is the percentage composition of fluorine in copper II fluoride?
37.42%
What are the primary industrial uses of copper II fluoride?
Wood preservation, electronics, and other industries